how does a car battery work physics
A car battery is a plastic box divided into six cells that is filled with an electrically conductive sulfuric acid solution called an electrolyte. A battery transfers initial currents of energy over to an alternate performance system of your vehicle an alternator.
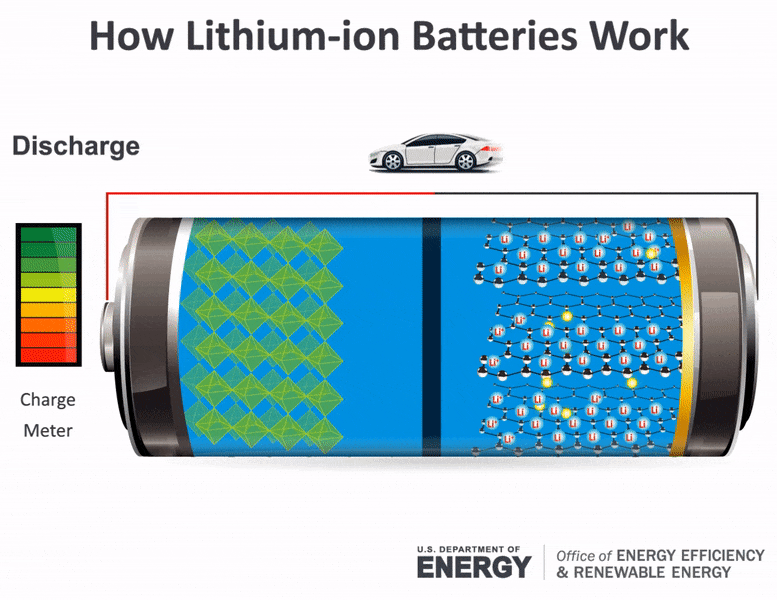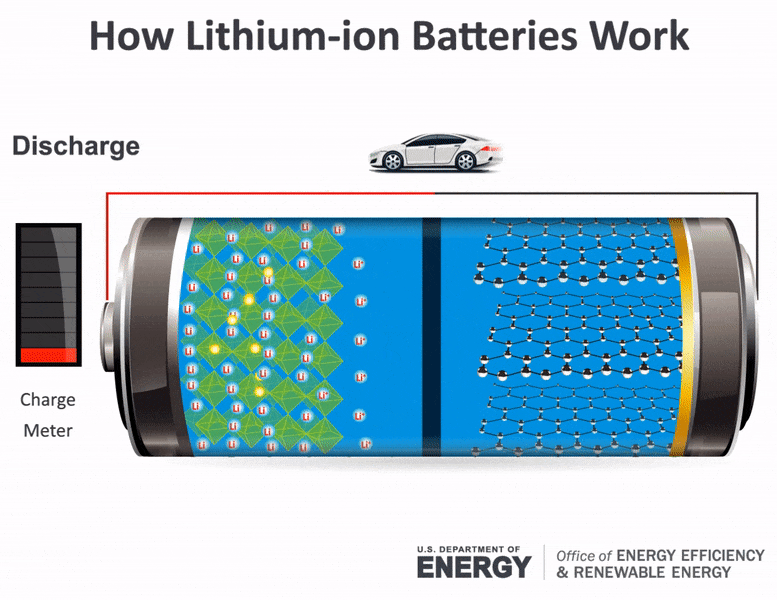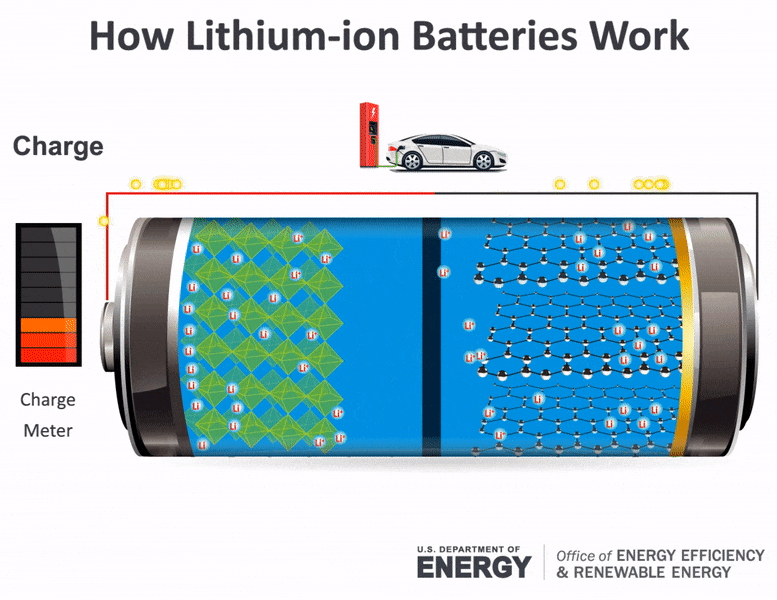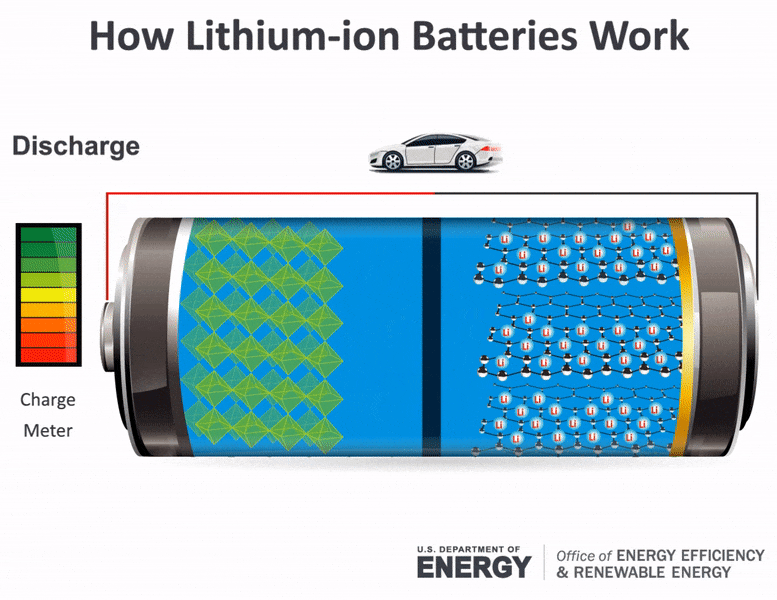
Science Made Simple What Are Batteries And How Do They Work
A battery starts up the ignition system of your vehicle.

. Definition of a Battery. A system such as a battery favors a state of lower enthalpy and it reaches that by two chemical reactions happening. This electrical power is delivered to the starter to crank the engine.
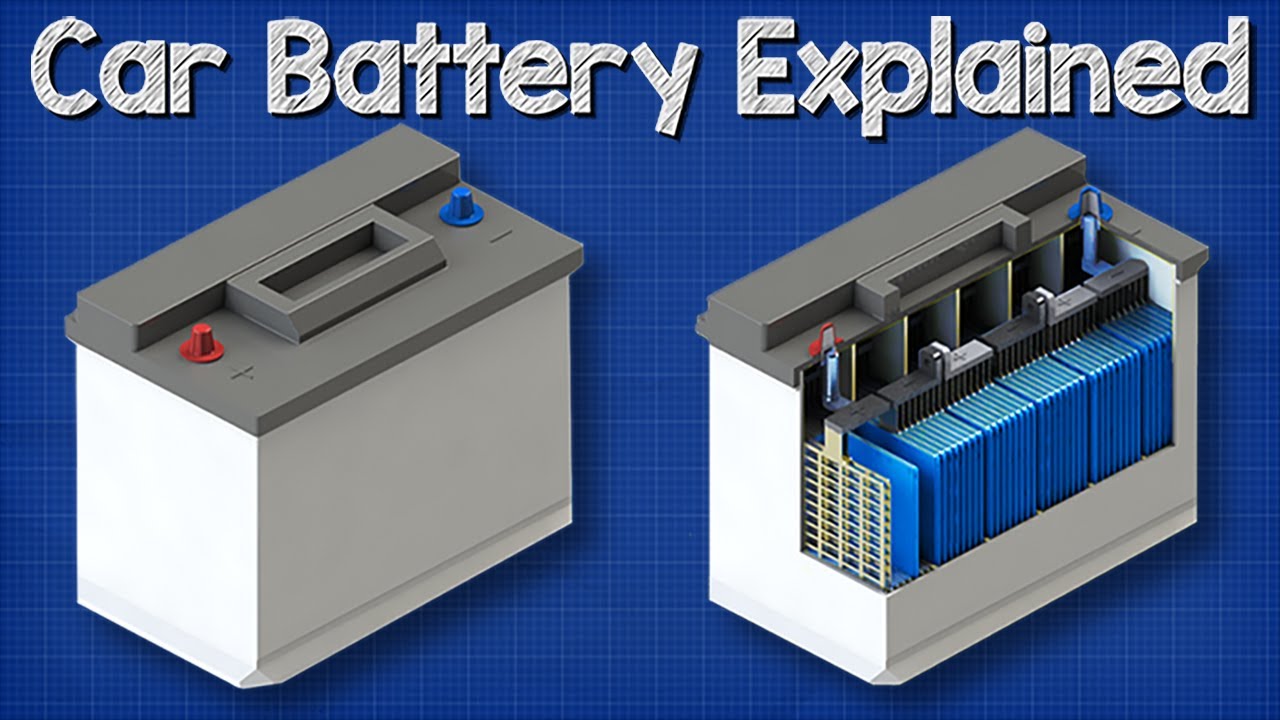
Typical batteries are powered by a chemical reaction. A car battery stores energy in chemical form and converts it into electrical energy. The plates are connected at the top by a cast-on strap that is welded to the plates.
In contrast the chemical reaction in a secondary cell is reversible. The negative plates of the battery are pure lead in a soft sponge-like state. A reaction at the positive electrode whereby ions receive electrons from the electrode.
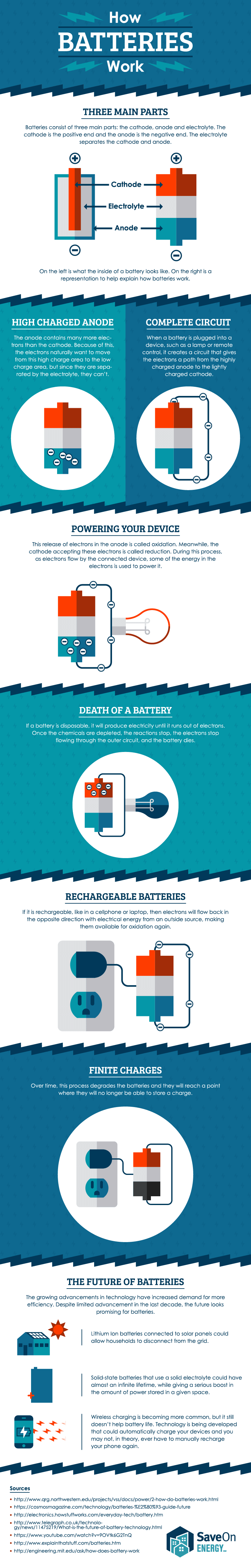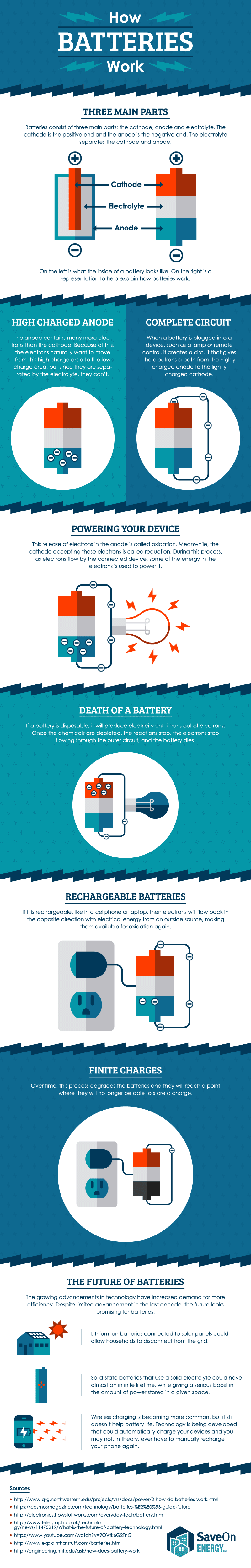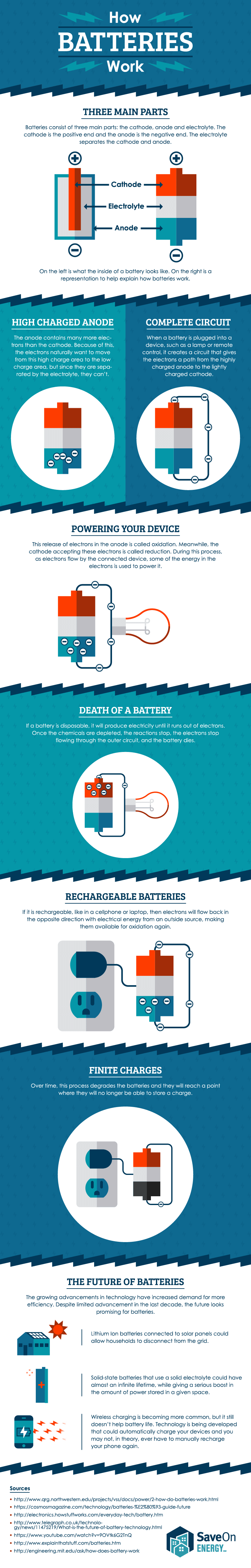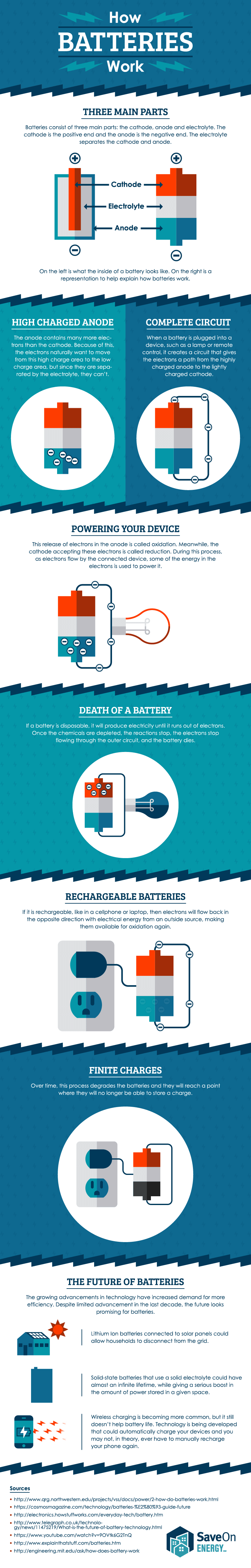
A cell consists of a negative electrode. A battery stores chemical material for conversion to electrical energy. A car battery is actually 6 smaller batteries that are lined up in series.
A battery keeps the energy system of your vehicle sustainable. Strictly speaking a battery consists of two or more cells connected in series or parallel but the term is generally used for a single cell. The main components for the SLI battery include six 2V cells hence 12V in total each with two platesgrids one lead and one lead dioxide and sulphuric acid which acts as.
Once the chemicals are exhausted the battery is effectively dead. With thanks to Squarespace for sponsoring this video. A battery works on the oxidation and reduction reaction of an electrolyte with metals.
Half of the plates are connected to each terminal. The formula for determining battery energy is. This chemical interacts with the batterys electrodes or metal plates containing lead and lead oxide to produce 12 volts of electricity.
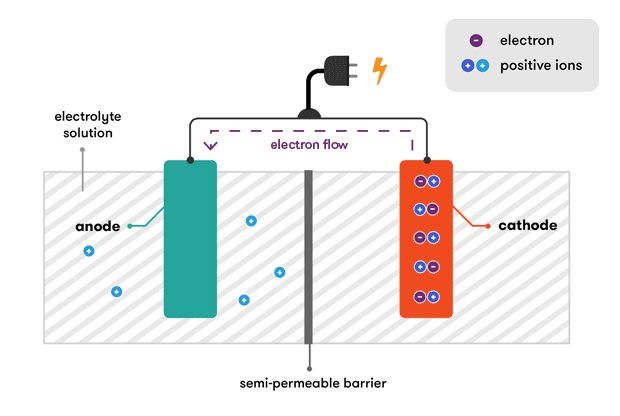
An electrolyte which. A battery is a device that stores chemical energy and converts it to electrical energy. How does a car battery work learn from the basics where we use and battery and how batteries work.
The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode. The battery also acts as a capacitor to smooth out current ripples and protect the vehicles sensitive onboard electronics. As a result the electrical difference between the cathode and anode starts increasing.
Upon receiving this signal the car battery converts chemical energy into electrical energy. A battery which is actually an electric cell is a device that produces electricity from a chemical reaction. Electricity supplied to the battery causes a chemical reaction that deposits extra lead on one set of plates.
Once the motor is running an alternator supplies current to recharge the battery. In this electro-chemical process four materials react with each other. There are 2 connectors that go out of the battery.
Heres how the conventional lead-acid liquid cell car battery worksThe purpose of a car battery is to provide electricity to start the engine spark for ign. Inside the battery are 3 important things. There are three main components of a battery.
This causes the voltages of each battery to add. Each cell contains a series of electrodes or plates. See full infographic Image credit.
The general way that a battery works is that when an electronic circuit is connected to the battery electrons are allowed to flow. All of the positive plates in a cell are. And the electrolyte which separates these terminals.
But the electrolyte keeps the electrons from going straight from the anode to the cathode within the battery. By Karl Tate Infographics Artist The final part of the battery the separator is fairly. A car battery is used mainly to start the motor.
The flow of electrons provides an electric current that can be used to do work. A few car battery companies include Diehard and Trojan. The chemical reaction that powers a primary cell is one way.
A set of connected cells is called a battery. The car battery has three basic tasks. After giving an initial burst of power to get the engine moving it outputs its energy in short bursts via its alternator.
The elements fit into the individual cells of each battery. After a short time the battery becomes fully recharged. When the reaction runs in its spontaneous direction the battery produces a.
Two terminals made of different chemicals typically metals the anode and the cathode. When the circuit is closed a wire connects the cathode and the anode the electrons will be able to get to. Battery Voltage Voltage refers to the amount of electrical potential your battery holds.
It adheres to the positive and negative battery grids. E Pt VIt Where I Current Expressed in amperes V Electromotive force expressed in volts. So a car has a rechargeable battery and a charging system to keep it topped up.
The positive plates of the battery are constructed from lead peroxide PbO2. The chemical reactions in a battery involve the flow of electrons from one material electrode to another through an external circuit. The plates within each cell are arranged in alternating layers for a total of 16 components.
The battery also provides power to the cars lights and other accessories. When the rechargeable battery is connected to an external power source the electrons starts moving in the reverse direction that is from the cathode to anode. The battery has pairs of lead plates immersed in a mixture of sulphuric acid and distilled water.
The negative cable connects to a common ground while the positive cable connects the battery to the starter motor and other necessary points on the vehicle. A reaction at the negative electrode whereby ions give up electrons at the electrode and 2. The paste is a lead oxide mixture that creates both lead dioxide and sponge lead.
Batteries come in two basic types. When two dissimilar metallic substances called electrode are placed in a diluted electrolyte oxidation and reduction reaction take place in the electrodes respectively depending upon the electron affinity of the metal of the electrodes. In a battery the only place to go is to the cathode.
Electrons repel each other and try to go to a place with fewer electrons. Two cables a negative and a positive connect the battery to the car.

Battery Working Principle How Does A Battery Work Electrical4u

How A Car Battery Works The Engineering Mindset

The Lead Acid Car Battery Schoolworkhelper

How Rechargeable Batteries Work Video Lesson Transcript Study Com
6 1 Electromotive Force Introduction To Electricity Magnetism And Circuits
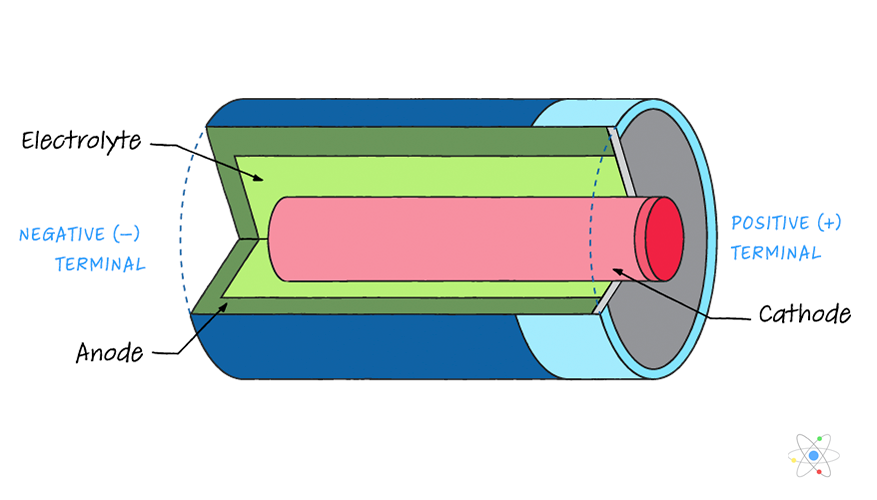
How Do Batteries Work Parts Types Terminology W Diagram
How Does A Car Battery Work Mach 1 Services
Electric Vehicle Battery Pollution

How A Car Battery Works Basic Working Principle Youtube

How A Car Battery Works The Engineering Mindset

Maintenance Of Lead Acid Battery Electrical4u

How A Car Battery Works The Engineering Mindset

How Batteries Work Physics Projects Science Projects Science Fair

Can You Run A Space Heater Off A Car Battery Heatertips

Relationship Between Battery Cold Cranking Amps And Capacity Math Encounters Blog
How Batteries Work An Animated Guide To The Science Of Batteries